Pcl5 Polar or Nonpolar
Polar square pyramidal Polar molecular geometry. PCl5 is nonpolar in nature because it has the symmetrical geometrical structure due to which the polarity of P-Cl bonds gets canceled by.

Dimethyl Sulphoxide Molar Mass Chemistry Boiling Point
Learn to determine if PCl5 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look an.
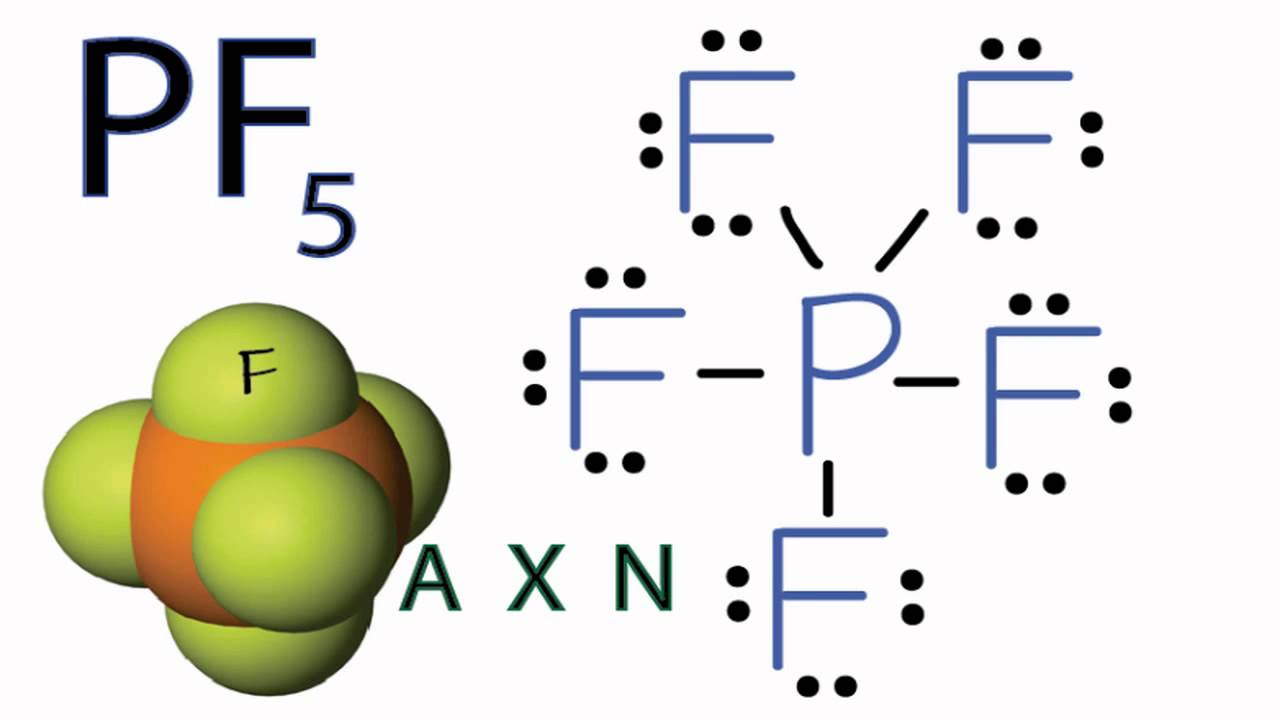
. The electron geometry of PCl 5 is also Trigonal bipyramidal. Now in the next step we have to check whether these C-H bonds are polar or nonpolar. Phosphorous pentachloride PCl 5 is a nonpolar molecule.
Polar see saw ICl5. The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure. Start studying Polar or Non-Polar.
Pcl5 polar atau nonpolar PERBEDAAN SENYAWA POLAR DAN NON P. Plus Cl is more electronegative than P in PCl5. This value is less than 04 which indicates that the bond between Carbon C and Hydrogen H is nonpolar.
As far as boiling point is concernedPCl3 does have a lower boining point than PCl5 because of the greater polarity as PCl3 has a trigonal pyramidal structure with a net dipole moment while. As all the five bonds P-Cl are symmetrical and the PCl5 molecule has a symmetrical geometry their bond polarity gets canceled with each other. The BONDS in PCl5 are POLAR because of Chlorines greater electronegativity compared to Phosphorus.
The net dipole moment in PCl. Polar trigonal bipyramidal SF4. Pcl5 Polar Or Nonpolar - 16 images - best overview is so2 polar or nonpolar science education and tutorials lewis dot structure of pcl5 phosphorous pentachloride.
And we also have to check the molecular geometry of hexane. Hence the bonds formed in this molecule are Polar Bonds. However the C-Cl bond is a polar covalent bond but the four bonds.
However the entire molecule is NONPOLAR because the Chlorines. All Exam Help Tips Free Latest Jobs NotificationsThe Latest Govt Jobs Notifications Jobs Alertment. Hey Guys In this video we are going to determine the polarity of Phosphorus Trichloride having a chemical formula of PCL3To know the polarity of this molec.
Learn vocabulary terms and more. Is CCl4 polar or non-polar. In the PCl 5 Lewis dot structure a total of 15 lone pairs and 5 bond pairs are present.
Because of this there. But the molecule PCl5 overall is a nonpolar chemical compound due to its Cl. The hybridization of phosphorous in PCl 5 is sp.
It has a symmetrical trigonal bipyramidal geometry with bond angles of 90 and 120. Hence each C-H bond is a nonpolar covalent bond. Is PCl5 polar or nonpolar.
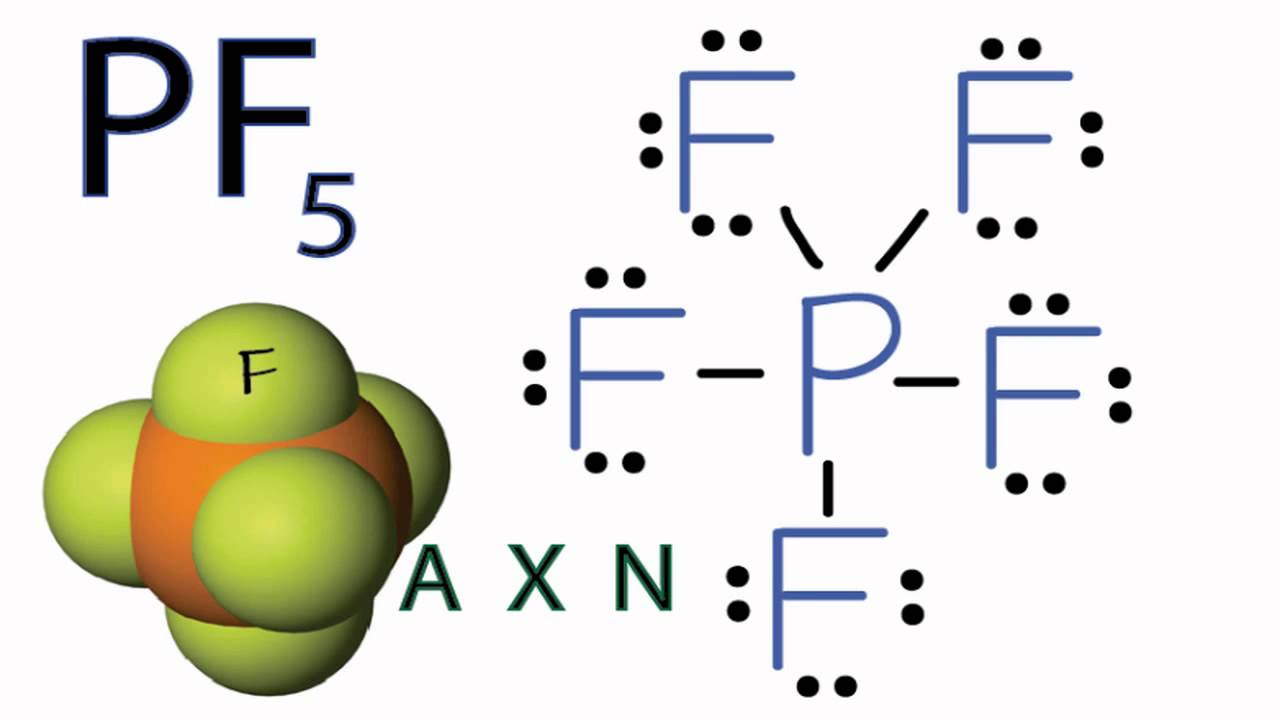
Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular

Science Coverage Is Xef4 Polar Or Nonpolar Molecular Geometry Covalent Bonding Polar

Comments
Post a Comment